Interoperable laboratory data are critical to ensure the timely and accurate delivery of laboratory test results for clinical decision-making and public health surveillance and monitoring. Interoperable exchange of data has also been shown to reduce costs and improve efficiency by limiting the ordering of potentially redundant tests.(1) Yet ensuring interoperability of laboratory data is a complex matter. Over 14 billion laboratory test results are ordered annually, and the flow of data cuts across many different entities and systems, and several health agencies are involved with its oversight.(2) Health information exchange organizations (HIEs/HIOs) provide a means to electronically share health care data, including laboratory results, across a given geographical area with HIOs’ participants, which can include a diverse array of health care providers as well as public health entities. Laboratories are key data contributors to HIOs and understanding their participation in these efforts provides a window into the completeness of data available to HIO participants. This data brief uses data from a 2023 national survey of HIOs to provide insights into lab participation in HIOs and how impediments in access to data by laboratories may limit the completeness of laboratory data made available to HIOs.
HIGHLIGHTS
- 4 in 5 HIOs nationwide make laboratory results available to participating organizations.
- Hospital-based laboratories share test results with, as well as view or receive data from, HIOs at higher rates than labs of other types.
- Of the third of HIOs that report impediments in access to data by laboratories, 1 in 5 reported that they were “not at all” able to overcome this difficulty to access data from labs.
- A frequent reason provided to HIOs by laboratories for limiting or refusing to share data is that they do not derive value as a data contributor only.
- A much larger portion (9 in 10) of HIOs perceive experiencing information blocking by independent laboratories (including commercial labs) compared to labs of other types.
An overwhelming majority of HIOs make laboratory results available, but the portion of HIOs with this data available varies by region.
FINDINGS
★ 4 in 5 HIOs nationwide make laboratory results available for participating organizations.
★ Among HIO respondents, HIOs made laboratory results available across 93% of hospital service areas (HSAs) in which they operate.
★ Using COVID-19 lab timeliness as a proxy for general timeliness of lab result data, most HIOs report receiving laboratory results either real-time or within 24 hours across different types of laboratories (Appendix Figure A1).
Figure 1: Percent of HIOs that make laboratory results available by hospital service area (HSA).
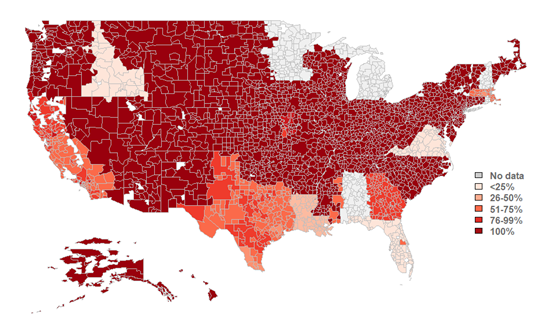
Source: National Survey of Health Information Exchange Organizations (2023).
Notes: N = 77 HIO respondents. This map represents HIO respondents that indicated the HSAs in which they operate and completed Q6.6 (relating to the availability of lab results). HSAs with “no data” refer to areas with no survey respondents to these questions; therefore, no metric could be calculated for these areas. See Data Sources and Methods for more information. See Appendix Table A1 for Survey Questions and Appendix Table A2 for a breakdown of the number of HIO respondents operating within each U.S. state.
According to HIOs, in 2023 hospital-based labs shared test results with HIOs and viewed or received data from HIOs at the highest rates.
FINDINGS
★ Physician office-based labs and independent labs had similar rates of providing test results to HIOs; however, physician office-based labs had twice the rate of viewing or receiving data from HIOs in comparison to independent labs.
★ In 2023, 71% of HIOs reported public health labs provided test results and about one-third reported that these labs viewed or received data from HIOs.
★ About half of HIOs reported that mobile labs (e.g., point of care labs for COVID-19) were not involved with their organization, and over a fifth reported that public health labs (25%) and independent labs (21%) were not involved with their organization.
★ HIOs less frequently reported that labs of each type view or receive data from their organization than they reported that these labs provided test results.
Figure 2: Percent of HIOs that report laboratory involvement in providing test results and viewing or receiving data from the HIO, by laboratory type, 2023.
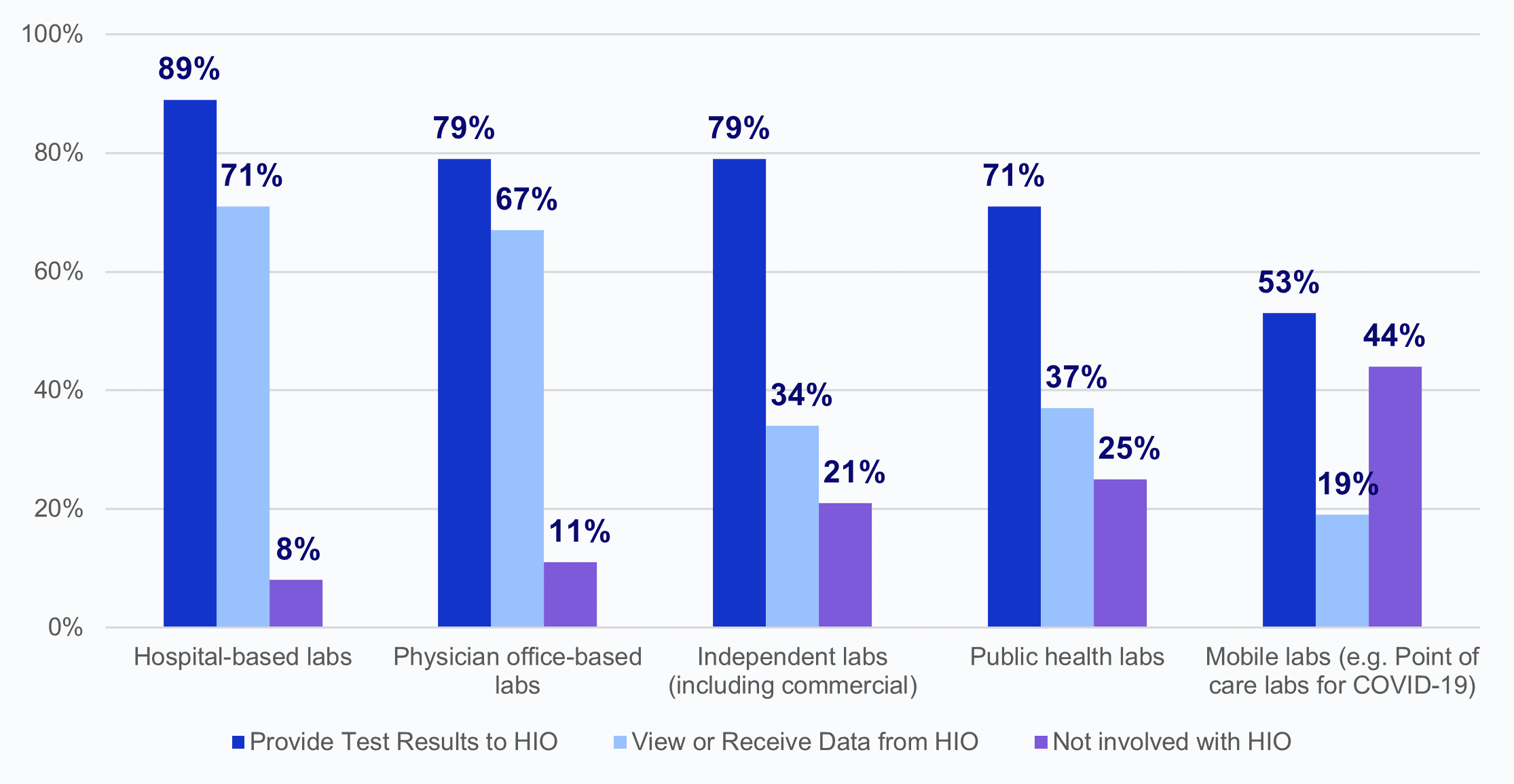
Source: National Survey of Health Information Exchange Organizations (2023).
Notes: Hospital-based labs N = 62 HIO respondents; physician office-based labs N = 57 HIO respondents; independent labs N = 56 HIO respondents; public health labs N = 51 HIO respondents; mobile labs N = 43 HIO respondents; and other labs N = 4 HIO respondents. Missing responses were excluded from the denominators: hospital-based labs N=15; physician office-based labs N=20; independent labs N=21; public health labs N=26; mobile labs N=34. Laboratories that fell into an “Other” category (N=4) were excluded from this figure because of high missingness (N=73); 75% of the HIOs that responded reported that “Other” labs were not involved with their organization. See Appendix Table A1 for Survey Questions.
Over one-third of HIOs report that laboratories have limited or refused to provide access, exchange, or use of electronic health information (EHI).
FINDINGS
★ Among HIOs that experienced impediments in access to data by laboratories, about 1 in 5 indicated that they were not at all able to overcome these difficulties to access lab data.
★ Nearly 4 in 5 HIOs that experienced an issue with access to data reported that they were able to overcome difficulties due to lab impediments “to a small extent” (22%) or “somewhat” (57%).
★ Notably, no HIOs reported that they had been able to “fully” overcome these difficulties to access data from laboratories and only 4% reported being able to overcome these difficulties “to a great extent.”
Figure 3: Percent of HIOs that experienced impediments in access to data by laboratories and the extent to which HIOs have been able to overcome these difficulties to access laboratory data.
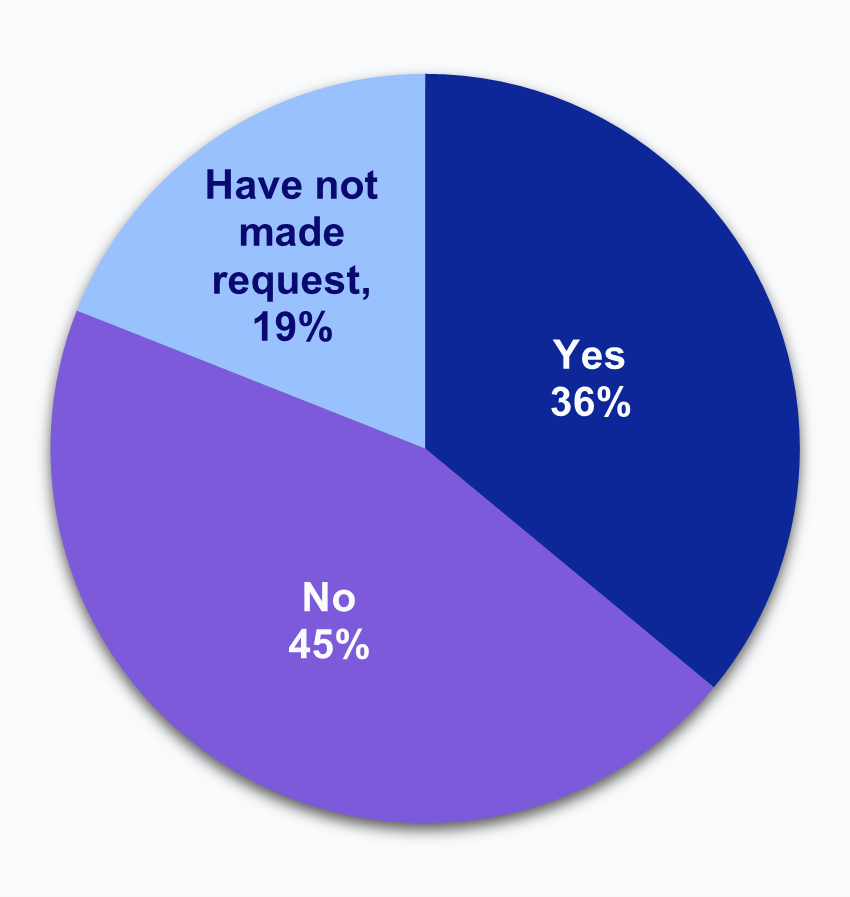
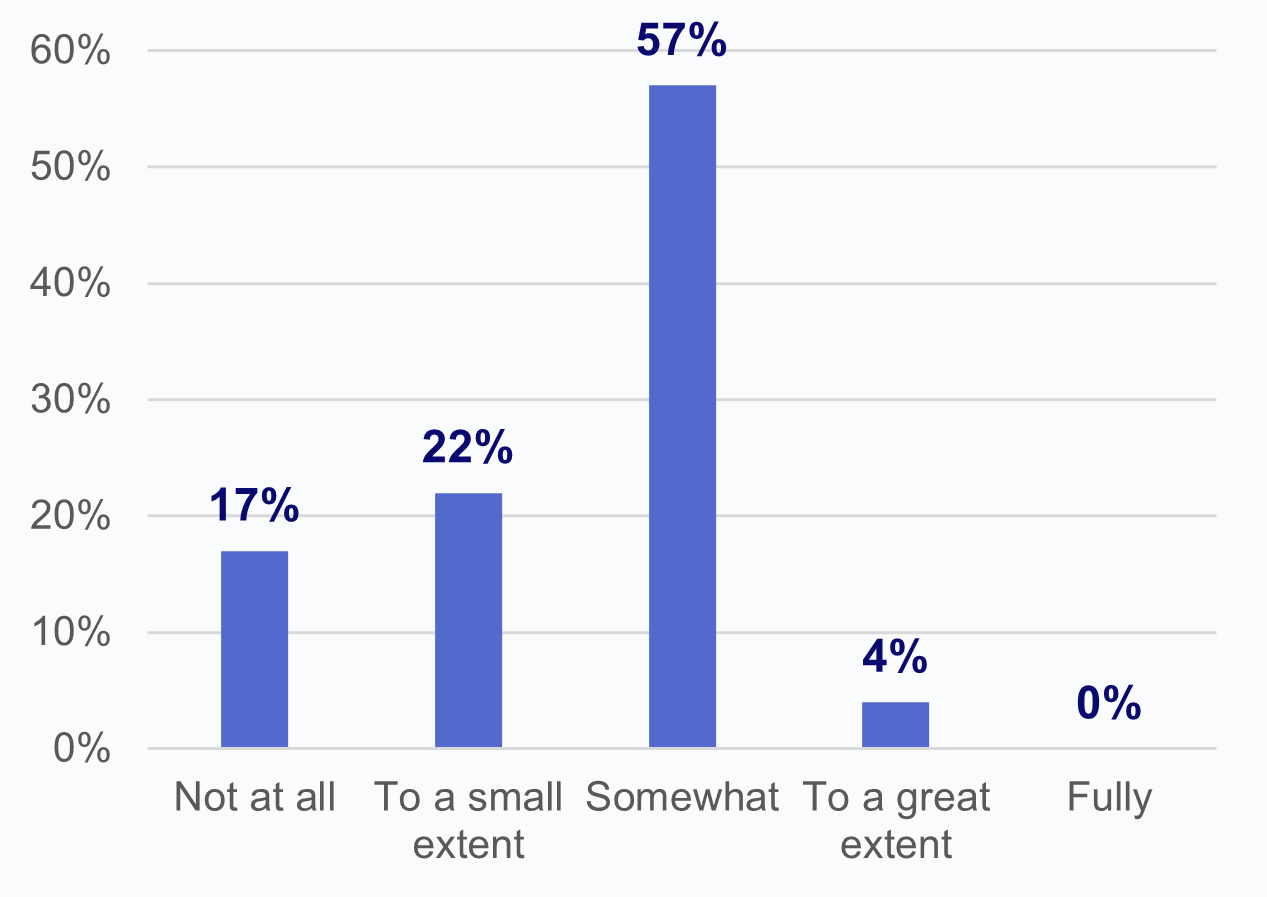
Source: National Survey of Health Information Exchange Organizations (2023).
Notes: N = 64 HIO respondents. N = 13 missing responses were excluded from the denominator. See Appendix Table A1 for Survey Questions.
Most HIOs reported that labs justified limiting or refusing to provide electronic health information because they do not derive value as a data contributor only.
FINDINGS
★ The most common reasons for not sharing data provided to HIOs who had experienced impediments in access to data by labs were that labs don’t derive value as a data contributor only (61%), labs are not obligated to engage in additional reporting after sharing results with the ordering provider (52%), and concerns about complex consent processes requiring multiple disclosure forms (48%).
★ No HIO reported that labs sought to limit or refuse to provide access to data due to their concerns with HIOs’ ability to perform patient matching, and only 4% of HIOs reported that labs expressed concerns with producing duplicate data.
★ Over half of HIOs that reported impediments in access to data by labs cited 2 or more reasons labs used as a basis for limiting or refusing to provide EHI (Appendix Figure A2).
Table 1: Reasons HIOs report that labs provided as the basis for limiting or refusing to provide HIE access to electronic health information, among HIOs that experienced impediments in access to data.
Which of the following reasons have laboratories used as the basis for limiting or refusing to provide electronic health information to your HIE? | |
|---|---|
Labs don’t derive value as a data contributor only | 61% |
Labs reporting obligation ends with returning result to ordering provider | 52% |
Labs need consent from each individual provider, resulting in your HIE having to execute multiple disclosure forms | 48% |
Role of CLIA or other federal regulations in restricting them from sending additional data | 30% |
Public health agencies (including emergency rules) do not mandate reporting to HIE | 30% |
Exchanging data with HIEs is not considered related to treatment, payment, or operations and would thus require patient consent | 22% |
Technological reasons/use of specific standards (convenient reason or wide spectrum of what labs are able to do) | 17% |
Fees associated with HIE participation | 17% |
Concerns with producing duplicate data | 4% |
Concerns with HIE’s ability to do patient matching | 0% |
Source: National Survey of Health Information Exchange Organizations (2023).
Notes: N = 23 HIO respondents. The denominator excludes HIOs that did not report experiencing impediments in access to data by laboratories (“No” responses N = 29, “Have not made request” responses N = 12, and missing responses N = 13). See Appendix Table A1 for Survey Questions.
HIOs most frequently reported that independent laboratories sought to limit or refuse access to electronic health information.
FINDINGS
★ Among HIOs that reported labs sought to limit or refuse access to electronic health information (36%), nearly all these HIOs (96%) reported independent laboratories, including commercial labs, did so.
★ A much smaller portion of HIOs reported that mobile labs (9%), public health labs (9%), and hospital-based labs (4%) sought to limit or refuse access to information.
★ No HIO reported physician office-based labs sought to limit or refuse access to information.
Figure 4: Among HIOs that reported labs sought to limit or refuse access to electronic health information, the percent of HIOs that experienced this impediment by laboratories of various types.
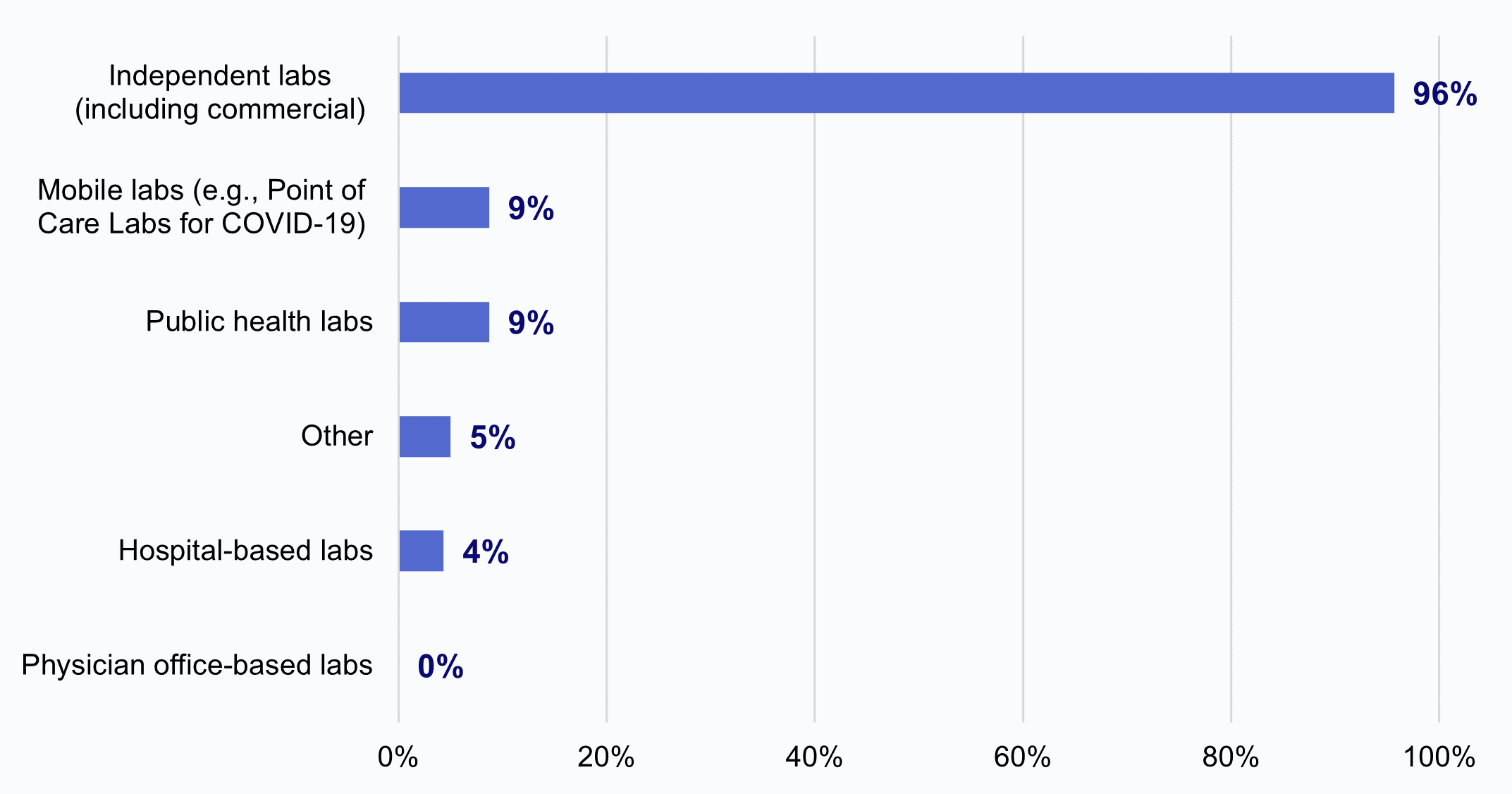
Source: National Survey of Health Information Exchange Organizations (2023).
Notes: N = 23 HIO respondents. The denominator excludes HIOs that did not report experiencing impediments by laboratories (“No” responses N = 29, “Have not made request” responses N = 12, and missing responses N = 13). See Appendix Table A1 for Survey Questions.
Two in five HIOs reported that commercial laboratories engaged in potential information blocking at least sometimes.
FINDINGS
★ Over one-third of HIOs reported that commercial labs rarely or never engaged in potential information blocking.
★ Notably, 1 in 4 HIOs indicated that they did not know whether commercial labs had engaged in potential information blocking.
Figure 5: HIOs’ perceptions of the frequency of potential information blocking by commercial laboratories.
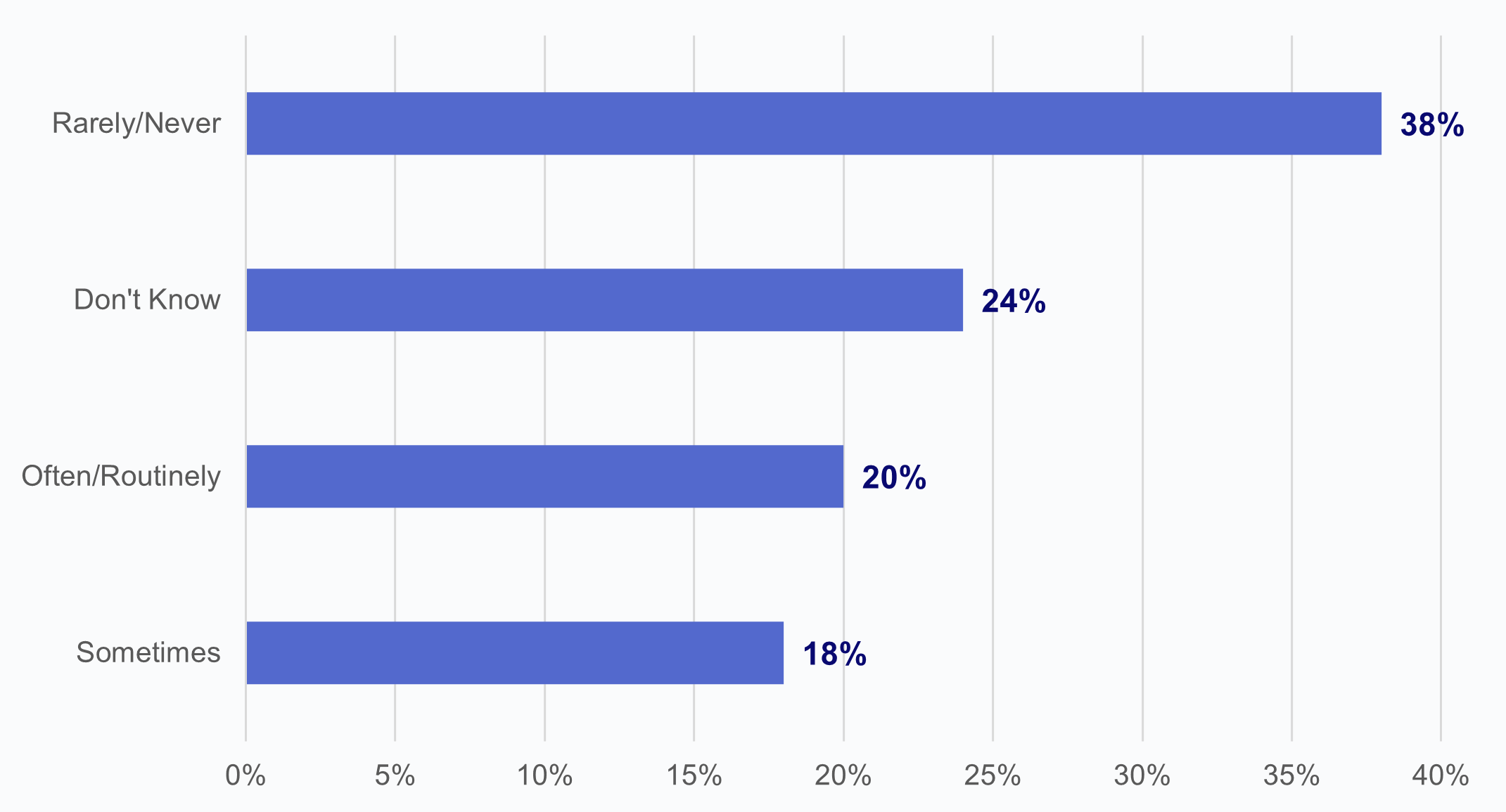
Source: National Survey of Health Information Exchange Organizations (2023).
Notes: N = 66 HIO respondents. Denominator represents HIOs who reported potential information blocking by commercial laboratories. See Appendix Table A1 for Survey Questions.
SUMMARY
HIOs offer participating health care providers within a geographical area a network for exchanging electronic health information, including laboratory results. We found that the vast majority of HIOs (nearly 4 in 5) reported making laboratory result data available to participating organizations, although the portion of HIOs that make this data available varies regionally by Hospital Service Area (HSA). Most HIOs reported receiving COVID-19 laboratory results from different lab types either in real-time or within 24, suggesting that the lab test data that HIOs have available are timely.
Although availability and timeliness of laboratory test results was relatively high across HIOs nationwide, the comprehensiveness and completeness of HIOs’ laboratory data varied by the extent to which laboratories provided these data to HIOs. Currently, laboratory data is commonly shared with providers through HL7v2 interfaces and laboratory portals which involve one-to-one connections directly with providers.(3) We found variation in the degree to which different types of laboratories share these data with HIOs. Based on HIOs’ survey responses, hospital-based, physician office-based, and independent labs share test results with HIOs at the highest rates; compared to public health labs and mobile labs. HIOs also reported that hospital-based and physician office-based labs view or receive data from HIOs at notably higher rates than other lab types. This is not altogether surprising given that hospitals and physicians may be key participants of HIOs, and thus derive value from HIOs. Overall, HIOs reported that labs view or receive data from their organization much less frequently than they reported that these same labs provide test results to the HIO. The limited engagement of laboratories with viewing or receiving data from HIOs may point to a potential missed opportunity. For example, laboratories could be leveraging HIO data to address issues such as missing demographic and contact information from HIOs, which surfaced during the COVID-19 pandemic as major problems that limited contact tracing and public health surveillance activities.(4) Additionally, this could serve as a means for laboratories to derive value from sharing data with HIOs.
Even among HIOs that receive laboratory test result data from labs, this is not without challenge. Just over one-third of HIOs report that laboratories have in general sought to limit or refused to provide access, exchange, or use of health information; while these instances represent impediments in access to data they cannot be qualified as acts of information blocking without a fact-based, case-by-case assessment of the circumstances.(5) The 21st Century Cures Act (Cures Act) generally prohibited information blocking and applied the law to health care providers (of which laboratories are included), health IT developers of certified health IT, and health information exchanges and networks (HIEs/HINs).(6) As of this writing, there are nine information blocking exceptions.(7) “Information blocking is a practice by an "actor" that is likely to interfere with the access, exchange, or use of electronic health information (EHI), except as required by law or specified in an information blocking.”(8) Although most HIOs (3 in 5) that reported experiencing impediments in access to data by laboratories indicated that they were at least "somewhat" able to overcome these difficulties to access data, notably, no HIOs reported that they had been able to “fully” overcome these difficulties to access data from laboratories and only 4% reported being able to overcome these difficulties “to a great extent.”
We also found that most HIOs reported experiencing impediments in access to data by independent labs, particularly commercial laboratories. Nearly all (96%) HIOs reported that they had experienced impediments in access to data by independent (including commercial) laboratories, compared to just 9% for mobile labs, 9% for public health labs, 4% for hospital-based labs, 0% for physician office-based labs, and 5% for labs of another type. Notably, these results may not necessarily reflect acts of information blocking by laboratories. However, impediments in access to data by commercial laboratories are especially problematic because commercial laboratories are responsible for processing a very high volume of test results nationally, and thus their limited participation may greatly impact the completeness of HIOs’ laboratory data.
Among HIOs that reported experiencing impediments in access to data by laboratories, 2 in 5 cited at least four or more reasons provided by labs for limiting or refusing to provide access, exchange, or use of electronic health information (Appendix Figure A2). The most cited reason given to HIOs by laboratories was that "labs don’t derive value as a data contributor only" (61%). If HIOs and laboratories could identify services and/or data that HIEs can provide to laboratories, as noted earlier, such as addressing information gaps, laboratories may be more enabled to share their data with HIOs. A 2020 Final Report on the National Survey on Health Information Exchange in Clinical Laboratories prepared by NORC at the University of Chicago points to a knowledge gap in laboratories' perspectives of the potential benefits of accessing patient information via HIOs, compounded by non-participant labs unfamiliarity with how HIO network payment models work.(8) This relates to the earlier finding that HIOs reported that laboratories' rates for viewing or receiving data from HIOs are less than their rates of providing data to HIOs. Both surveys acknowledge the untapped potential for labs to participate more in HIOs; indicating an opportunity for HIOs to extend informational resources to labs on how to bridge these perceived barriers to exchanging data, while highlighting the benefits to prospective laboratories, commercial and otherwise.
Other reasons commonly cited by HIOs for laboratories refusing to provide data related to interpretation of regulations and the perceived need for consent before sharing data with HIOs. About half of HIOs indicated that "labs’ reporting obligation ends with returning result to ordering provider" (52%).Other related reasons included the belief that labs needed consent from each individual participating provider before sharing the data with the HIO; labs citing Clinical Laboratory Improvement Amendments (CLIA) and other federal regulations as restricting them from sending additional data to HIOs; and labs indicating that exchanging data with HIOs is not considered related to treatment, payment, or operations and thus would require patient consent. HIOs’ reports echo findings from the 2020 NORC Final Report which found some commercial laboratories referenced their understanding of CLIA regulations to mean laboratory result reporting responsibilities are to the ordering provider exclusively.(3) This interpretation may also lead laboratories to exclude data sharing between HIOs as a Health Insurance Portability and Accountability Act (HIPAA) treatment, payment, or operation (TPO) activity. These findings indicate that certain laboratories could benefit from additional education and clarification regarding how regulations govern the sharing of data with HIOs, and when some kind of patient permission may be necessary.
Having gone into production in late 2023, the Trusted Exchange Framework and Common Agreement (TEFCATM) is a potential avenue by which some of the documented impediments to laboratory data exchange via networks could be reduced.(9) TEFCA has three goals: (1) to establish a universal governance, policy, and technical floor for nationwide interoperability; (2) to simplify connectivity for organizations to securely exchange information to improve patient care, enhance the welfare of populations, and generate health care value; and (3) to enable individuals to gather their health care information. Ensuring HIOs, laboratories, and others in the healthcare industry understand TEFCA’s goals, components, and larger commitment to the Cures Act will aid in more efficient and effective electronic health information exchange at a nationwide scale.
DEFINITIONS
Interoperability: The ability of a system to exchange electronic health information with and use electronic health information from other systems without special effort on the part of the user.
Health information exchange: Refers to physicians’ ability to electronically send or receive patient health information from other providers outside their medical organization using an EHR system or query any patient health information from sources outside of their medical organization.
Health Information Exchange Organization (HIE/HIO): Local, regional, and state organizations operating in the United States that supported live electronic health information exchange across unaffiliated entities. This does not include local proprietary or enterprise networks.
Information blocking: Considered a practice by an "actor" that is likely to interfere with the access, exchange, or use of electronic health information (EHI), except as required by law or specified in an information blocking exception.
DATA SOURCES AND METHODS
This brief reports results from the 2023 National Health Information Organization (HIO) Survey, which was fielded from January to July 2023. Our goal was to survey all local, regional, and state organizations operating in the United States as of March 1, 2022, that supported live electronic health information exchange across unaffiliated entities. We initially invited leadership from 135 HIOs to participate in the survey. Among this initial group, 45 HIOs were determined to be ineligible for the survey, including 26 that were no longer in service, 4 that did not pursue or no longer pursued live exchange, and 14 that had merged with another exchange or operated under a broader exchange. 77 of the 90 remaining organizations met our inclusion criteria and completed the survey, resulting in an 86% response rate.
Respondents had the option to complete the survey via online survey tool Qualtrics© or over the phone if they preferred that to the online platform. Non-respondents received follow-up emails and one phone call to encourage response. As with prior years, respondents were offered a $50 gift card for completing the survey or a $10 gift card if they were screened out. The responding HIOs operated in 47 states and the District of Columbia.
For this survey, the Office of the Assistant Secretary for Technology Policy/Office of the National Coordinator for Health (hereafter ASTP) partnered with the University of California at San Francisco (UCSF) and Civitas Networks for Health (Civitas) to examine how HIOs continue to evolve and the role they play in enabling interoperability, as it relates to the broader scope of various policies such as the Health Information Technology for Economic and Clinical Health (HITECH) Act of 2009 and the Trusted Exchange Framework and Common Agreement (TEFCA). UCSF, under contract with ASTP, led the development, testing, and fielding of the instrument. Civitas, through a separate contract, informed the development of the instrument and facilitated testing of the draft instrument. The UCSF team was led by Julia Adler-Milstein, PhD, Professor and Director of the Division of Clinical Informatics and Digital Transformation at UCSF. Dr. Adler-Milstein led the six previous national surveys of HIOs since 2007. ASTP has funded the 2023 and 2020 fielding of the survey.
DATA AVAILABILITY
Data is available upon request. Please contact ASTP_Data@hhs.gov.
REFERENCES
- Pylypchuk Y, Johnson C. New EHR certification requirements and their association with duplicate tests and images. J Am Med Inform Assoc. 2022 Jul 12;29(8):1391-1399. Available from: https://pubmed.ncbi.nlm.nih.gov/35640013/
- Centers for Disease Control and Prevention. Strengthening Clinical Laboratories. Division of Laboratory Systems. 2018. Available from: https://www.cdc.gov/csels/dls/strengthening-clinical-labs.html
- NORC. Final Report: Office of the National Coordinator for Health Information Technology (ONC) National Survey on Health Information Exchange in Clinical Laboratories. NORC at the University of Chicago. 2020. Available from: https://www.norc.org/content/dam/norc-org/pdf2024/onc-national-survey-health-information-exchange-clinical-labs-final-report.pdf
- Chambers S, Eike K, Jessup L, Perlie LaVerne. STAR HIE Program Helps Unlock Powerful Public Health Data in West Virginia. Office of the National Coordinator for Health Information Technology. 2022. Available from: https://www.healthit.gov/buzz-blog/public-health/star-hie-program-helps-unlock-powerful-public-health-data-in-west-virginia.
- Office of the National Coordinator for Health Information Technology. Frequently Asked Questions. Office of the National Coordinator for Health Information Technology. 2022. Available from: https://www.healthit.gov/faq/when-would-delay-fulfilling-request-access-exchange-or-use-ehi-be-considered-impediments-under
- United States of America. 21st Century Cures Act: Interoperability, Information Blocking, and the ONC Health IT Certification Program. [Internet]. Washington, D.C.: Federal Register; 2020 [cited 2024 Apr 10]. Available from: https://www.federalregister.gov/documents/2020/05/01/2020-07419/21st-century-cures-act-interoperability-information-blocking-and-the-onc-health-it-certification.
- Office of the National Coordinator for Health Information Technology (ONC). Information Blocking Exceptions Fact Sheet [Internet]. Washington, D.C.: ONC; 2024 Apr [cited 2024 Aug 30]. Available from: https://www.healthit.gov/sites/default/files/2024-04/IB_Exceptions_Fact_Sheet_508_0.pdf
- Office of the National Coordinator for Health Information Technology. Information Blocking. Office of the National Coordinator for Health Information Technology. 2024. Available from: https://www.healthit.gov/topic/information-blocking.
- Office of the National Coordinator for Health Information Technology. Top Takeaways from the TEFCA Recognition Event. Office of the National Coordinator for Health Information Technology. 2023. Available from: https://www.healthit.gov/buzz-blog/interoperability/top-takeaways-from-the-tefca-recognition-event
ACKNOWLEDGEMENTS
The authors are with the Office of Standards, Certification, and Analysis, within the Office of the Assistant Secretary for Technology Policy. The data brief was drafted under the direction of Mera Choi, Director of the Technical Strategy and Analysis Division, Vaishali Patel, Deputy Director of the Technical Strategy and Analysis Division, and Wesley Barker, Chief of the Data Analysis Branch with subject matter expertise from Sara Armson, Daniel Healy, Cassie Weaver, and Rachel Nelson.
SUGGESTED CITATION
Strawley C, Larson J, Patel V. Laboratory Interoperability Through Health Information Exchange Organizations. Office of the Assistant Secretary for Technology Policy. Data Brief: 74. September 2024.
APPENDIX
Appendix Figure A1: Timeliness of COVID-19 laboratory test results by type of laboratory.
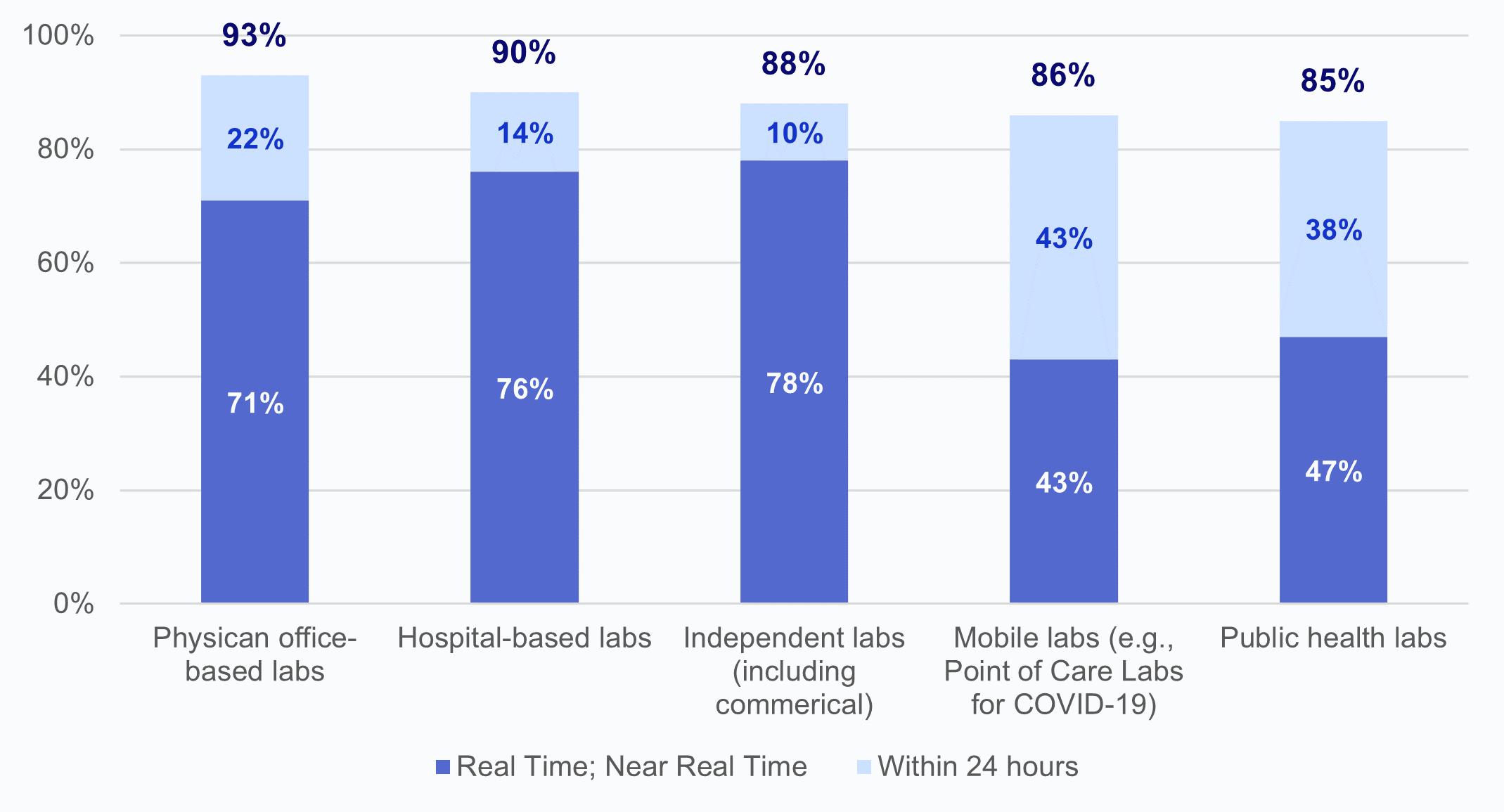
Source: National Survey of Health Information Exchange Organizations (2023).
Notes: Physician office-based labs N = 41 HIO respondents and missing responses N = 36; hospital-based labs N = 49 HIO respondents and missing responses N= 28; independent labs N = 41 HIO respondents and missing responses N = 36; mobile labs N = 21 HIO respondents and missing responses N= 56; public health labs N = 34 HIO respondents and missing responses N = 43; and other labs N = 1 HIO respondents and missing responses N = 76. Laboratories that fell into an “Other” (N=1) category were excluded from this figure due to high missingness; 100% of HIOs reported timely delivery of lab test results “within 24 hours” by laboratories of the type “Other.” See Appendix Table A1 for Survey Questions.
Appendix Table A1: Survey questions used in laboratory interoperability analysis.
Question Text | Response Options |
|---|---|
Lab Participation in COVID-19 Relevant HIE | |
Please report whether each type of stakeholder is involved in your HIE in the following ways: | Respondents were prompted to complete each of the following stakeholder options:
selecting all that apply among the following HIE options:
|
How timely are COVID-19 test results that you typically receive? | Respondents were prompted to complete each of the following stakeholder options:
corresponding to one of the following timeliness options:
|
In general, have laboratories sought to limit or refused to provide access, exchange, or use of electronic health information (e.g., laboratory results)? | Yes | No | Have not made request |
What types of laboratories have sought to limit or refused to provide access, exchange, or use of electronic health information? | Respondents were prompted to select all that apply among the following options:
|
Which of the following reasons have laboratories used as the basis for limiting or refusing to provide electronic health information to your HIE? | Respondents were prompted to select all that apply among the following options:
|
To what degree have you been able to overcome these difficulties to access data from laboratories? | Not at all | To a small extent | Somewhat | To a great extent | Fully |
Implementation and Use of Standards | |
Which types of clinical and other health-related information are made available by your HIE (as part of a clinical document or as a structured data element)? | Respondents were prompted to select all that apply among the following Laboratory-Related Information options:
|
Implementation and Use of Standards | |
Among other types of stakeholders, to what extent have you observed information blocking behaviors? | Respondents were prompted to complete each of the following stakeholder options:
corresponding to the following frequency options:
|
Source: National Survey of Health Information Exchange Organizations (2023).
Notes: Eight questions analyzed from HIO Survey.
Appendix Table A2: HIO respondents operating in each state.
State | Percent of HSAs with HIO Respondents | Number of HIO Respondents with Operations in the State |
|---|---|---|
Alabama | 0 | 0 |
Alaska | 100 | 2 |
Arizona | 100 | 2 |
Arkansas | 100 | 2 |
California | 100 | 11 |
Colorado | 94 | 3 |
Connecticut | 100 | 2 |
Delaware | 100 | 3 |
District of Columbia | 0 | 1 |
Florida | 100 | 3 |
Georgia | 100 | 4 |
Hawaii | 100 | 1 |
Idaho | 100 | 1 |
Illinois | 100 | 3 |
Indiana | 100 | 1 |
Iowa | 100 | 2 |
Kansas | 100 | 5 |
Kentucky | 100 | 3 |
Louisiana | 100 | 4 |
Maine | 100 | 1 |
Maryland | 100 | 3 |
Massachusetts | 100 | 3 |
Michigan | <1 | 3 |
Minnesota | 0 | 2 |
Mississippi | 100 | 2 |
Missouri | 100 | 5 |
Montana | 100 | 1 |
Nebraska | 100 | 2 |
Nevada | 100 | 1 |
New Hampshire | 0 | 0 |
New Jersey | 100 | 6 |
New Mexico | 100 | 2 |
New York | 71 | 7 |
North Carolina | 100 | 1 |
North Dakota | 100 | 2 |
Ohio | 100 | 2 |
Oklahoma | 100 | 2 |
Oregon | 100 | 1 |
Pennsylvania | 100 | 4 |
Rhode Island | 0 | 0 |
South Carolina | 100 | 2 |
South Dakota | 100 | 2 |
Tennessee | 100 | 1 |
Texas | 100 | 6 |
Utah | 100 | 2 |
Vermont | 100 | 1 |
Virginia | 100 | 1 |
Washington | 100 | 2 |
West Virginia | 100 | 1 |
Wisconsin | 100 | 1 |
Wyoming | 100 | 2 |
Source: National Survey of Health Information Exchange Organizations (2023).
Notes: N = 77 HIO respondents.
Appendix Figure A2: Count of laboratory reasons for limiting or refusing to provide electronic health information cited by HIOs.
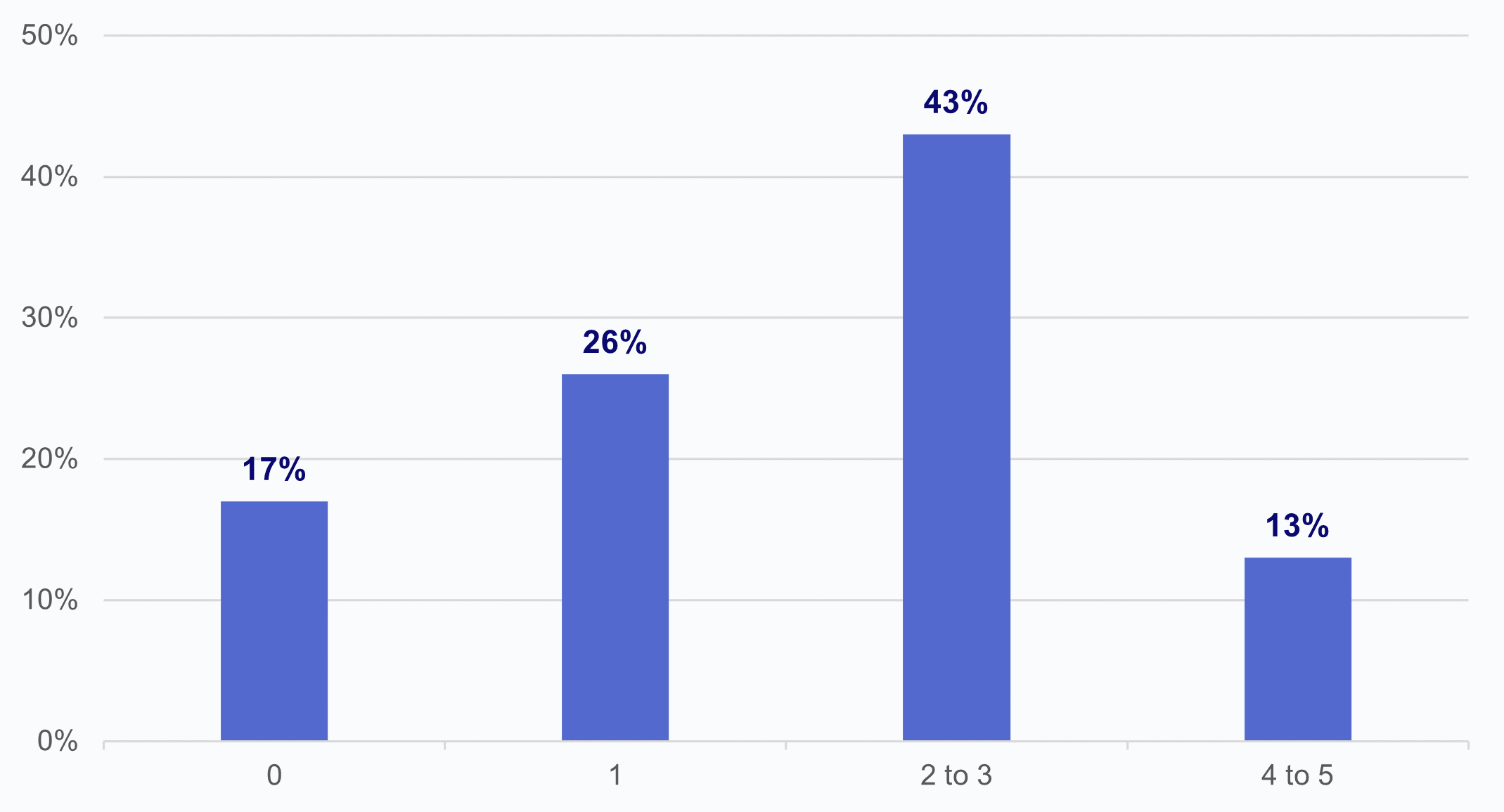
Source: National Survey of Health Information Exchange Organizations (2023).
Notes: N = 23 HIO respondents. The denominator includes respondents that indicated that laboratories “have sought to limit or refused to provide access, exchange, or use of electronic health information (e.g., laboratory results).” See Appendix Table A1 for Survey Questions.


